FDA Drug Label Section Checker
Click a section above to see its importance and what to look for.
Every time you or someone you care about takes a prescription drug, there’s a detailed label behind it-written by the FDA-that holds the full story of what the drug does, who it’s safe for, and what could go wrong. But most people never read it. Not because they don’t care, but because it looks like a legal document written in tiny print. The truth? You don’t need to read every word. You just need to know where to look.
The FDA Drug Label Isn’t Just Paper-It’s a Safety System
The U.S. Food and Drug Administration doesn’t just approve drugs. It also controls how the information about those drugs is presented. Since 2006, all prescription drug labels in the U.S. must follow a strict format called the United States Prescribing Information (USPI). This isn’t optional. It’s the law. And it’s designed so that doctors, pharmacists, and patients can find critical safety info fast.
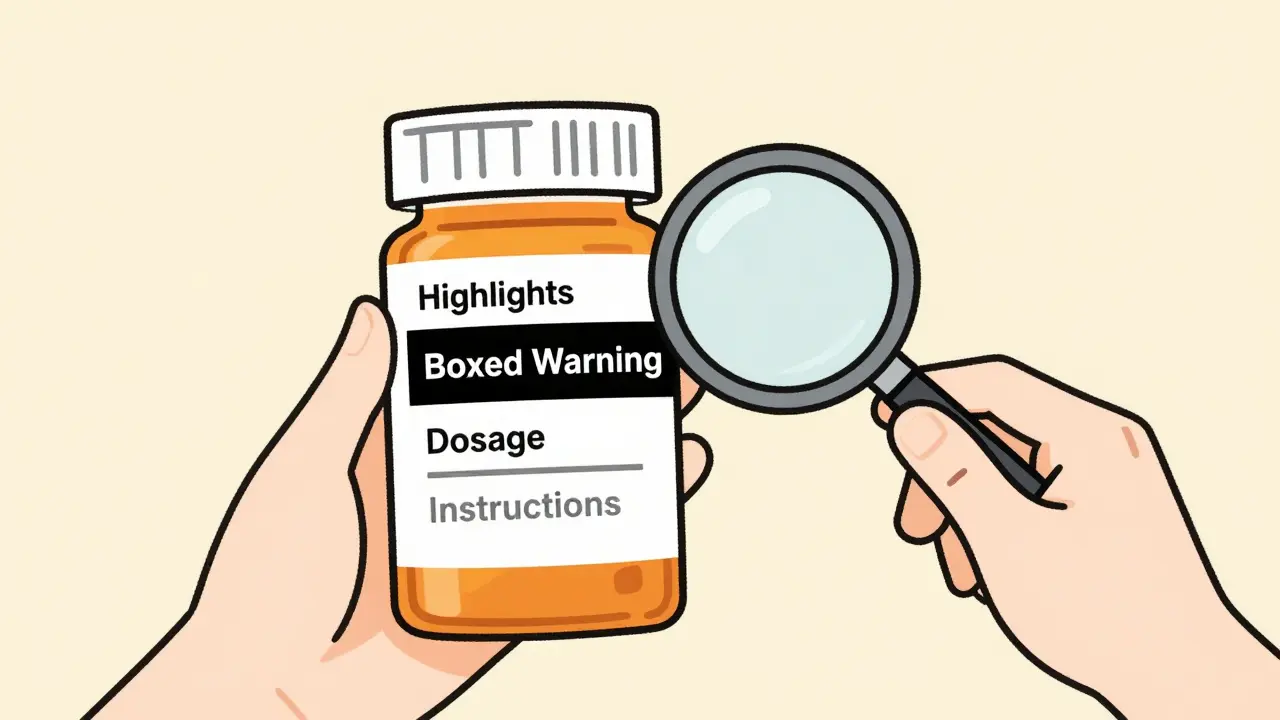
The label has three main parts: the Highlights, the Table of Contents, and the Full Prescribing Information (FPI). The Highlights are just half a page. They’re meant to give you the most urgent details-like who should take the drug, the most serious risks, and how to take it. But here’s the catch: the Highlights are not the whole story. They say right on them: "This is not complete information. See full prescribing information." If you only read this part, you’re missing half the picture.
Section 1: Indications and Usage-What This Drug Is Actually For
This is where you find out if the drug is even meant for your condition. It doesn’t just say "for high blood pressure." It lists the exact medical conditions it’s approved to treat, including off-label uses that have been studied and accepted by the FDA. For example, a drug might be approved for Type 2 diabetes but also shown to help with weight loss in clinical trials. That’s listed here.
Look for phrases like "Established Pharmacologic Class"-these tell you how the drug works in the body. Is it an ACE inhibitor? A selective serotonin reuptake inhibitor? That helps you understand why it’s being prescribed and how it might interact with other meds.
Section 2: Dosage and Administration-How Much, How Often, How to Take It
This section is where mistakes happen. Too many people guess their dose. But this part tells you exactly: starting dose, maximum dose, how to adjust for kidney or liver problems, whether to take it with food, and if it should be swallowed whole or split. The FDA updated this section in March 2024 to make dosing for elderly or impaired patients clearer.
For example, if you have reduced kidney function, the label might say: "Reduce dose by 50% if eGFR is less than 30 mL/min." That’s not vague. That’s a precise instruction. If your doctor doesn’t mention this, ask why.
Section 5: Warnings and Precautions-The Red Flags
This is the most important section for safety. It’s broken into two parts: the Boxed Warning and the rest of the warnings.
The Boxed Warning-also called a "Black Box Warning"-is the FDA’s strongest safety alert. It’s in a thick black border at the top of the label. If a drug has one, it means serious injury or death has been linked to it. Common examples: increased risk of suicide with antidepressants, heart failure with certain diabetes drugs, or severe allergic reactions. This isn’t a suggestion. It’s a red light.
Beneath that, you’ll find other warnings: risk of bleeding, liver damage, infections, or changes in mood. These aren’t just "possible side effects." They’re known risks that doctors must consider before prescribing.
Section 6: Adverse Reactions-What Could Go Wrong
This section lists side effects, but not just any side effects. These are the ones that showed up in clinical trials. Each one has a frequency: very common (over 10%), common (1-10%), uncommon (0.1-1%), rare (under 0.1%).
Don’t panic if you see "headache" listed as common. That’s normal. But if you see "severe hypotension" listed as uncommon, and you’re already on blood pressure meds, that’s something to flag with your doctor. The key is context: how often does it happen? And how serious is it?
Section 7: Drug Interactions-What Not to Mix With This Drug
This is where many people get hurt. The label lists every known interaction with other drugs, supplements, or even foods. For example, grapefruit juice can make some cholesterol drugs dangerously strong. St. John’s Wort can cancel out birth control. Blood thinners like warfarin interact with over 100 different substances.
Pharmacists spend hours checking this section before filling a prescription. You should too. If you take more than three medications, write them all down and go through this section one by one. Don’t assume your doctor knows everything you’re taking. Many patients forget to mention vitamins or herbal teas.
Section 8: Use in Specific Populations-Is It Safe for You?
Is the drug safe if you’re pregnant? Breastfeeding? Over 65? A child? This section breaks it down. For example, some antidepressants are known to pass into breast milk and affect infant weight gain. Some diabetes drugs aren’t approved for kids under 10. And older adults often need lower doses because their bodies process drugs slower.
If you fall into one of these groups, this section is your lifeline. Don’t skip it. If your doctor says, "It’s fine," ask them to show you the part of the label that supports that.
Section 16: How Supplied / Storage and Handling-The NDC Code and More
This section looks boring, but it’s critical. It lists the exact drug strength, form (pill, liquid, injection), and the 10-digit National Drug Code (NDC). That code is like a barcode for your medication. It’s used by pharmacies, insurers, and the FDA to track every pill made.
Why does this matter? Because look-alike, sound-alike drugs cause 12.7% of all dispensing errors, according to the Institute for Safe Medication Practices. If your pill looks different than last time, check the NDC. If it changed, ask why. Also, this section tells you how to store the drug-some need refrigeration, others must stay dry. Improper storage can make a drug useless or even dangerous.
Section 17: Patient Counseling Information-What Your Provider Should Tell You
This section is written for patients. It tells your doctor exactly what to say when handing you the prescription. It includes plain-language advice like: "Take this on an empty stomach," "Avoid alcohol," "Call your doctor if you notice new bruising."
But here’s the problem: only 38.2% of providers actually use this section during patient visits, according to a 2024 national audit. That means you might be missing vital safety tips. If your doctor doesn’t mention anything from this section, ask: "Is there anything I should know about taking this that’s not obvious?" Then show them Section 17.

The Recent Major Changes Section-Don’t Ignore Updates
Since 2018, every FDA-approved drug label must include a "Recent Major Changes" section. It lists exactly which parts of the label were updated in the last six months. Maybe a new warning was added. Maybe the dose changed. Maybe a new interaction was found.
On average, labels get updated every 14.3 months. That means if you’ve been taking a drug for two years, its label has probably changed at least once. If you’re refilling a prescription, check this section. It’s often at the very beginning of the label. If you don’t see it, ask your pharmacist for the latest version.
How to Use This in Real Life
Here’s a simple 3-step plan for anyone taking a new prescription:
- Find the Boxed Warning and Highlights. If there’s a black box, understand what it means before taking the drug.
- Check Section 2 (Dosage) and Section 7 (Interactions). Make sure your other meds and health conditions are accounted for.
- Look at Section 17. Ask your provider: "Did you read the patient counseling part?" If they say no, request it.
For patients managing chronic conditions, keep a printed copy of the full label in your medication file. Update it every time you refill. You’ll be more informed than 90% of other patients.
Why This Matters More Than You Think
Medication errors are the third leading cause of death in the U.S., according to Johns Hopkins. Many of those errors happen because people didn’t understand the label. The FDA didn’t design this system to confuse you. It was built to protect you.
But protection only works if you use it. You don’t need to be a doctor. You just need to know where to look. The next time you get a new prescription, take five minutes. Find the label online at DailyMed.nlm.nih.gov. Read the Boxed Warning. Check the dosage. Look at the interactions. You might just avoid a hospital visit.
What’s Changing in 2025 and Beyond
The FDA is working on a new "Patient-Focused Labeling Initiative"-set to roll out draft guidelines in mid-2025. The goal? Make labels easier for patients to understand without losing medical accuracy. That means more plain language, visual icons, and maybe even QR codes linking to video instructions.
But until then, the current system is your best tool. It’s detailed. It’s regulated. It’s updated. And it’s free. All you have to do is read it.
What is the Boxed Warning on a drug label?
The Boxed Warning, also called a Black Box Warning, is the strongest safety alert the FDA requires. It appears in a thick black border at the top of the drug label and highlights the most serious risks-like death, life-threatening reactions, or severe organ damage. If a drug has one, it means clinical evidence shows this risk is real and significant. Never ignore it.
Can I rely only on the Highlights section of the FDA drug label?
No. The Highlights section is designed to give you a quick overview, but it’s intentionally incomplete. It says so right on the page: "This is not complete information." Critical details about dosing, interactions, and long-term risks are only in the Full Prescribing Information. Relying only on Highlights can lead to dangerous misunderstandings.
Where do I find the National Drug Code (NDC) on a drug label?
The NDC is in Section 16: How Supplied / Storage and Handling. It’s a 10-digit number broken into three parts: labeler code, product code, and packaging code. This code uniquely identifies your exact drug, strength, and packaging. Always check it when you refill a prescription to make sure you’re getting the same medication.
Why does the FDA require Structured Product Labeling (SPL)?
SPL is the FDA’s required electronic format for drug labels, based on XML standards. It ensures every drug label is machine-readable so electronic health records, pharmacy systems, and drug databases can pull accurate, up-to-date information automatically. Without SPL, systems like EHRs couldn’t reliably warn doctors about drug interactions or dosage limits.
How often are FDA drug labels updated?
On average, FDA drug labels are updated every 14.3 months. Updates happen when new safety data emerges-like a new side effect, interaction, or population restriction. All updates must be reported within 30 days. The "Recent Major Changes" section at the top of the label tells you what was changed in the last six months. Always check this before refilling a prescription.



Comments
13 Comments
Jane Lucas
just read the boxed warning and call it a day. if your doc prescribes it, they already did the work.
Nikki Thames
It is imperative to underscore that the United States Prescribing Information (USPI) constitutes a legally codified framework, the integrity of which is non-negotiable. To neglect the Full Prescribing Information (FPI) is to engage in a form of medical negligence that endangers not only oneself, but also the broader public health infrastructure. The FDA’s structural rigor must be honored with corresponding intellectual rigor.
James Bowers
The notion that patients should independently parse FDA labels is not merely impractical-it is a dangerous abdication of professional responsibility. Physicians are trained for this. Pharmacists are trained for this. To expect laypersons to interpret clinical trial data, pharmacokinetic adjustments, and boxed warnings is to institutionalize medical illiteracy under the guise of empowerment.
Olivia Goolsby
Have you ever stopped to think that the FDA doesn’t actually want you to understand this? The ‘Highlights’ section? A distraction. The ‘Recent Major Changes’? A cover-up. The NDC code? A tracking tool for Big Pharma to monitor your consumption patterns. They don’t want you to know that 73% of drug interactions are suppressed in the initial filings-only revealed after lawsuits. And that QR code they’re planning for 2025? It’s not for education-it’s for surveillance. They’re building a pharmacological panopticon, and you’re handing them the keys by reading the label like it’s a cookbook.
Monika Naumann
In India, we do not have such elaborate labeling systems. Our patients rely on physicians, not documents. This American obsession with self-diagnosis through regulatory text is not only inefficient-it is culturally alien. Why would a patient in rural Bihar read a 50-page PDF when their local pharmacist, who has known their family for decades, tells them what to do? The West confuses bureaucracy with safety.
Elizabeth Ganak
lol i just screenshot the pill bottle and send it to my aunt who’s a nurse. she tells me if it’s safe. why overcomplicate it? :)
Nicola George
So you’re telling me the government spent millions to design a label so complex that only a pharmacist with a PhD can decode it… and then they expect the average person to read it like a novel? Cute. I’ll take my chances with the pharmacist’s scribbled note on the bag. At least it’s in English.
Liz Tanner
my grandma reads the whole thing every time she gets a new script. she highlights the warnings, writes down the NDC, and brings it to her appt. she’s 82 and has never had a bad reaction. you don’t need to be a doctor-you just need to care enough to look.
Babe Addict
you’re all missing the point. The USPI is just the FDA’s attempt to shift liability onto patients. The real issue is that drug companies pay to influence the labeling structure. That ‘Boxed Warning’? Probably got watered down after 3 rounds of lobbying. And don’t even get me started on how the ‘Recent Major Changes’ section is buried in the XML metadata of the SPL. Only 0.7% of patients even know SPL exists. This isn’t transparency-it’s obfuscation with a FDA stamp.
Satyakki Bhattacharjee
People think medicine is science. It is not. It is power. The label is a tool to control. If you read it, you become a slave to paper. Better to trust your doctor, your faith, and your body.
Kishor Raibole
One must observe, with a certain degree of philosophical detachment, that the very architecture of the FDA drug label-structured, standardized, and solemnly regulated-represents a triumph of bureaucratic rationalism over human intuition. Yet, in its meticulousness, it paradoxically alienates the very population it seeks to protect. Is it safety, or is it spectacle? The patient, in this grand theater of pharmacology, is neither protagonist nor spectator-but a footnote in the footnote.
Elizabeth Alvarez
Did you know that the FDA allows drug companies to delay updating the label for up to 90 days after a new safety study is published? And that’s not even the worst part. The ‘Patient Counseling Information’ section? It’s written by the same pharma marketers who paid for the clinical trials. They pick the words. They pick the tone. They pick what you’re told. The whole thing is a marketing brochure dressed up as law. I’ve seen labels where the boxed warning says ‘risk of death’ but the patient section says ‘some people may feel a bit dizzy.’ That’s not a label. That’s a lie with a barcode.
Miriam Piro
Okay but what if the FDA is being manipulated by the same people who run the WHO? 🤔 I mean, think about it-every single drug label has the same structure. Same sections. Same jargon. What if this isn’t about safety… what if it’s about control? Like, they want us to think we’re empowered by reading the label, but really, they’re training us to trust the system. And once we trust the system… we stop asking questions. And once we stop asking questions… we stop rebelling. 💀👁️🗨️
Write a comment