When a medicine batch gets released, it doesn’t just go out the door because production hit its quota. Someone had to say yes - and that someone must not be the same person pushing for faster output, lower costs, or fewer delays. That’s the whole point of a Quality Assurance Unit (QU). In regulated manufacturing - especially in pharma, medical devices, and nuclear - independence isn’t a nice-to-have. It’s the line between a safe product and a dangerous one.
What Exactly Is a Quality Assurance Unit?
A Quality Assurance Unit is a formal, separate team with one job: to make sure everything made meets the rules. Not just the rules on paper, but the ones that keep people alive. They don’t make the product. They don’t run the machines. They don’t answer to the production manager. Their only boss is the truth - and the regulator. The FDA laid this out clearly in 2006: under a quality system, the QU, the manufacturing team, and the product development group must stay independent. Why? Because if the same person who’s under pressure to meet daily output targets also decides whether a batch is safe to ship, the pressure wins. Always. And when that happens, patients pay the price. This isn’t theory. In 2024, 68% of FDA warning letters cited failures in QU independence. That’s up from 29% in 2020. The trend isn’t slowing. In fact, the European Commission’s 2024 update to EudraLex made it even clearer: quality units must never be organizationally subordinate to production departments - under any circumstances.How Independence Actually Works in Practice
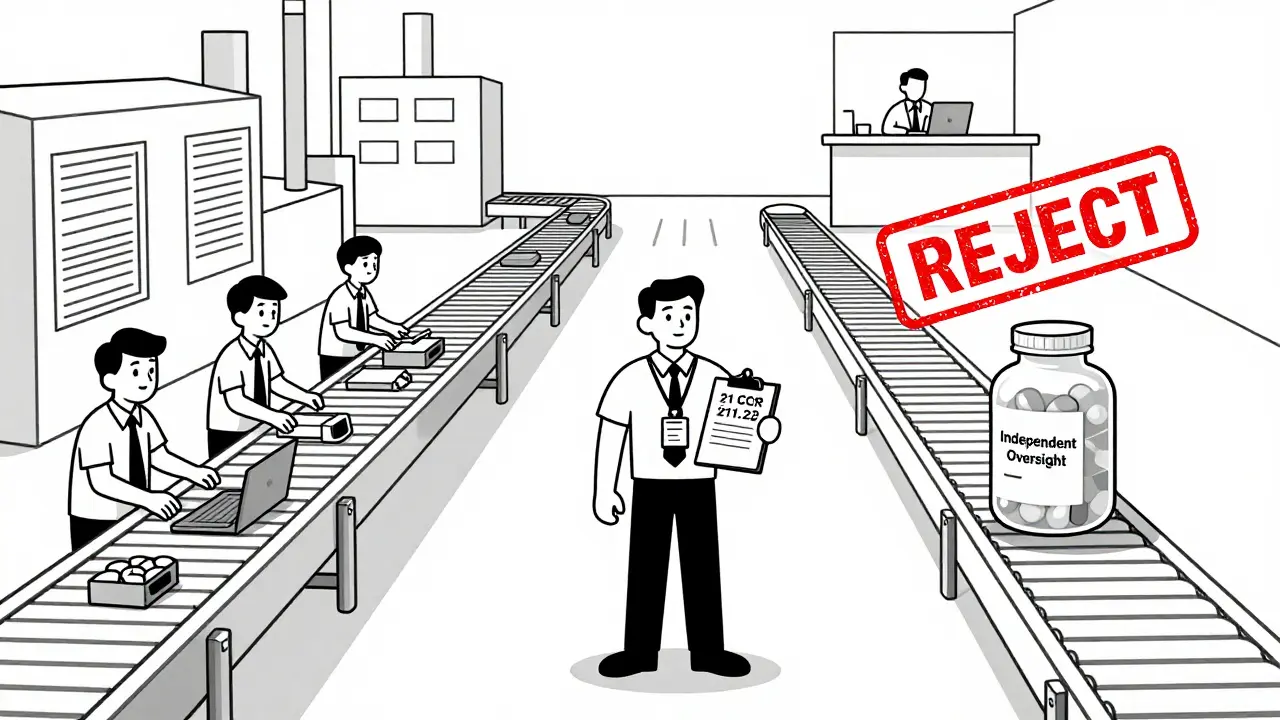
Independence doesn’t mean the QU sits in a locked room with no contact with production. It means they have the authority to say no - and the power to back it up. Under 21 CFR 211.22, the QU has the legal responsibility to approve or reject:- Incoming raw materials
- Containers and closures
- In-process samples during production
- Final packaging and labeling
- Every single batch of finished product
- The QU reports directly to the CEO or Board of Directors, not to the plant manager
- QU staff have their own budget, separate from production
- They can initiate a quality hold without needing approval from anyone in manufacturing
- They review and sign off on all procedures - not just review them
The Cost of Not Being Independent
The biggest mistake companies make? Trying to save money by combining roles. “We’re small,” they say. “One person can handle both production and quality.” That’s a trap. In 2024, 42% of FDA warning letters issued to facilities with fewer than 50 employees cited QU independence failures. That’s more than double the rate in larger companies. Why? Because in small teams, roles blur. The person who’s running the line also signs off on the batch. The production manager is also the quality manager. The pressure to deliver becomes the same pressure to approve. One Reddit user, ‘QualityAssurancePro,’ described how their company tried this in early 2024. The production manager took on QA duties during a restructuring. Three months later, two critical deviations slipped through - both were released without proper investigation. The batch went out. The company got a warning letter. The customer lost trust. The cost? Far more than the salary of a second person. Even worse: when the QU doesn’t have real authority, people start “rubber stamping.” They sign off because they don’t want to be the one who delays things. FDA data shows that facilities with a QU-to-production staff ratio below 1:15 have 3.2 times more repeat deviations. That’s not a coincidence. It’s a system failure.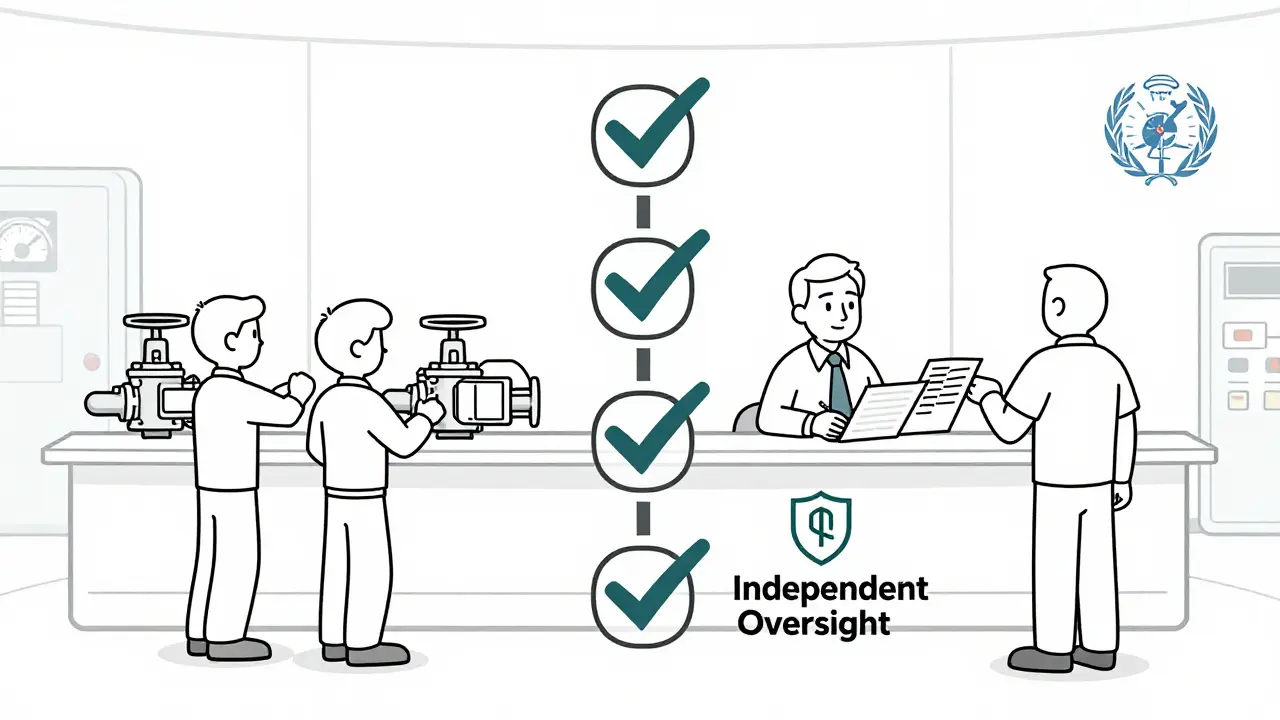
How Different Industries Handle It
Pharmaceuticals and nuclear energy both demand independence - but they do it differently. In the U.S., the FDA takes a hard line. The QU must be completely separate. No shared reporting lines. No dual roles. Even in digital manufacturing, where AI makes real-time decisions, the FDA’s 2025 draft guidance says the system must still be designed so that quality decisions can’t be overridden by production logic. In Europe, the EMA is a little more flexible. ICH Q10 says independence must be ensured, but doesn’t require full organizational separation - as long as there are clear mechanisms to prevent bias. In practice, though, most EU companies still go with full separation because it’s easier to prove. Nuclear facilities use a four-layer model:- Peer checking - operators verify each other’s work
- Functional oversight - senior managers monitor their teams
- Independent oversight - the QU, fully separate
- External oversight - regulators, IAEA, WANO
What Skills and Structure Make a QU Effective?
A QU isn’t just a group of people with “QA” in their title. It’s a team with specific skills and structure. According to the 2024 Pharma Industry Salary Survey, QU staff average 8.2 years of experience. That’s not accidental. They need to understand:- Regulatory requirements (GMP, 21 CFR, EU Annex 1)
- Statistical process control (78% of QU staff use this daily)
- How to say no - and how to stand their ground (65% need formal conflict resolution training)
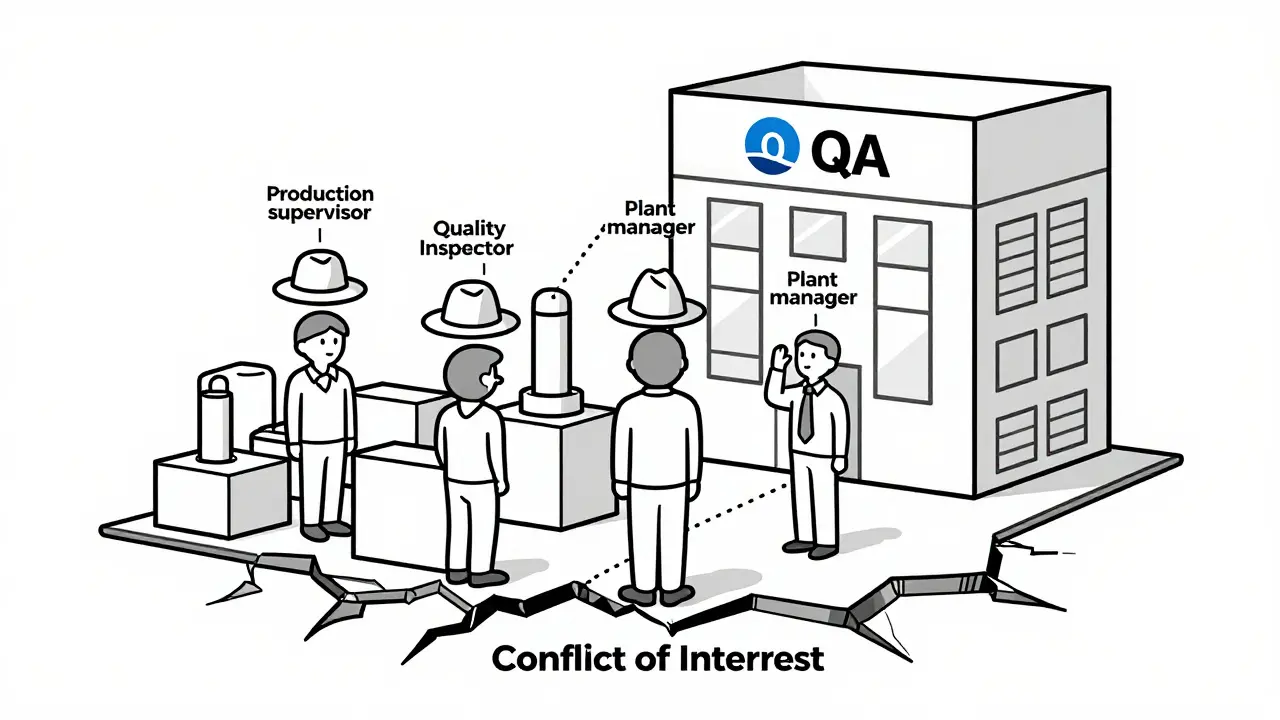
Small Companies and the Third-Party Solution
If you’re a small manufacturer with 30 people, hiring a full QU team isn’t feasible. But that doesn’t mean you can skip independence. The solution? Third-party quality oversight services. This market is growing at 14.2% per year. Companies outsource their QU functions to certified external firms that provide trained auditors, batch reviewers, and compliance monitors - all completely separate from your production team. It’s not cheaper. But it’s safer. And regulators are starting to accept it. In fact, the FDA now explicitly allows third-party oversight as long as the arrangement is documented, audited, and the external team has the same authority as an internal QU.What’s Next for Quality Assurance Units?
Digital manufacturing is changing the game. AI now predicts defects before they happen. Sensors monitor temperature, pressure, and humidity in real time. But here’s the catch: if the AI is trained on data from production teams who’ve been pressured to approve marginal batches, it learns to approve marginal batches. The FDA’s 2025 draft guidance on digital manufacturing is trying to fix that. It’s not about removing AI - it’s about separating the decision logic. The AI can flag anomalies. But only the QU - independent, human, accountable - can decide whether to release the batch. The future isn’t about more automation. It’s about clearer boundaries. Even in a world of algorithms, the human element of independent oversight remains irreplaceable.Final Thought: Independence Isn’t a Cost - It’s Insurance
Building a truly independent QU isn’t about bureaucracy. It’s about protecting lives. Every time you let production pressure influence a quality decision, you’re gambling with someone’s health. The data doesn’t lie. Companies with independent QUs have fewer failures, faster resolutions, and higher inspection success rates. They also avoid the reputational and financial damage of a recall or warning letter. If you’re in manufacturing - especially regulated manufacturing - there’s only one right answer: keep quality separate. Not because it’s hard. Not because it’s expensive. But because it’s the only way to make sure what you produce is safe.Can a production manager also serve as the Quality Assurance Unit lead?
No. Regulatory agencies like the FDA and EMA explicitly prohibit this. If the same person is responsible for both meeting production targets and approving product quality, a conflict of interest exists. The QU must have separate reporting lines, typically to the CEO or Board, and must have the authority to halt production without approval from production leadership. FDA warning letters from 2024 show that 68% of independence violations involved this exact scenario.
What happens if the Quality Assurance Unit doesn’t have the authority to reject batches?
If the QU can’t reject batches, it’s not a true Quality Assurance Unit - it’s just a paperwork department. Without rejection authority, the unit loses all regulatory credibility. In practice, this leads to batch releases that don’t meet specifications, increased risk of recalls, and almost certain FDA or EMA warning letters. The FDA considers this a critical violation of GMP, and facilities with this flaw are flagged for immediate corrective action.
How big should a Quality Assurance Unit be?
There’s no universal number, but ISPE benchmarks suggest 8-12% of total manufacturing staff. For a facility with 100 employees, that’s 8-12 QU staff. Smaller ratios - like 1:15 or worse - correlate with 3.2 times more repeat deviations, according to FDA data. The key isn’t just headcount, but authority: even a small team with direct CEO access and clear rejection power is more effective than a large team that needs approval to say no.
Can small manufacturers afford an independent Quality Assurance Unit?
Yes - but not necessarily by hiring full-time staff. Many small manufacturers use third-party quality oversight services, which are growing at 14.2% annually. These firms provide trained auditors, batch reviewers, and compliance experts who operate independently from your production team. The FDA accepts this model as long as the third party has documented authority to approve or reject batches and reports directly to your leadership. It’s often more cost-effective and far safer than trying to combine roles internally.
Is QU independence required outside of pharmaceuticals?
Yes. While the strongest rules are in pharmaceuticals and medical devices, nuclear energy, aerospace, and even some food manufacturing sectors require independent oversight. The IAEA mandates it for nuclear facilities, and ISO 13485 (for medical devices) requires quality functions to be free from undue influence. Even in less regulated industries, companies that maintain independent quality oversight see fewer defects, faster problem resolution, and stronger customer trust.


Comments
11 Comments
Neil Ellis
Man, this post hit different. I’ve seen QA teams get squeezed till they squeak - and it’s never the batch that pays the price, it’s the kid on the other side of the world who gets a tainted vial. This isn’t just compliance - it’s moral architecture. We build systems to protect people, not to hit quarterly targets. If your QA unit reports to production, you’re not making medicine. You’re making excuses.
Daphne Mallari - Tolentino
While the underlying premise is undeniably sound, one must interrogate the epistemological foundations of regulatory independence. The FDA’s rigid bifurcation model assumes a Cartesian separation between production and quality - a false dichotomy in complex adaptive systems. True quality emerges from systemic integration, not bureaucratic silos. The ICH Q10 framework, though more nuanced, is often misread as permissive when it is, in fact, prescriptive about functional autonomy.
Furthermore, the conflation of ‘independence’ with ‘organizational separation’ risks institutionalizing inefficiency. The most effective QUs I’ve observed operate with embedded subject-matter experts who retain dual reporting - but with a veto power that cannot be overridden. The data cited here, while compelling, conflates correlation with causation. It’s not the reporting line that matters - it’s the cultural capital of the QA lead.
Alec Amiri
Oh wow, another ‘quality over profit’ sermon. Newsflash: companies aren’t charities. If you’re spending 12% of your workforce on QA, you’re not a manufacturer - you’re a bureaucracy with a factory attached. I’ve worked at a 30-person med device shop. One guy did QA and production. We had zero recalls. Zero warnings. We just didn’t make junk. Maybe if you stopped overcomplicating things, you wouldn’t need a whole department to say ‘no’.
Also, third-party QA? That’s just outsourcing your conscience. Who checks the checker? The same people who check the FDA? LOL.
Sarvesh CK
It is fascinating to observe how the principle of independence, though rooted in Western regulatory traditions, finds parallel expressions across diverse cultural and industrial contexts. In Indian pharmaceutical manufacturing, particularly in family-run units, the role of the elder or founding member often serves as an informal but deeply respected quality gatekeeper - not because of formal authority, but because of moral weight and generational trust. The challenge lies not in replicating the FDA model, but in adapting its essence: the non-negotiable sanctity of the final decision point. When production pressure becomes the dominant narrative, even the most well-intentioned systems collapse. What is required is not merely structural separation, but a cultural reorientation - where saying ‘no’ is celebrated, not punished.
Moreover, the notion that small manufacturers cannot afford independent oversight assumes a binary choice: full-time staff or nothing. This ignores the possibility of cooperative QA networks - regional alliances of small firms sharing a rotating QA lead, funded collectively and governed by mutual accountability. Such models already exist in Kerala and Gujarat, and they outperform many larger, isolated operations.
Hilary Miller
This. So much this. I’ve been in rooms where QA was told to ‘just sign it.’ I didn’t. I quit. No regrets.
Margaret Khaemba
Wait - so if the QA unit can’t reject a batch, does that mean they’re just a glorified notary? Like, they stamp papers but can’t stop the train? That’s wild. I feel like I’ve seen this in my cousin’s job at a food plant - they had a ‘quality team’ but the plant manager signed off on everything. One batch of mayo had mold. No one got fired. Just a ‘sorry, customer got sick’ email. This isn’t just about pharma - it’s about trust. If the people who make the stuff also decide if it’s safe… we’re all just rolling the dice.
Malik Ronquillo
68% of warning letters? That’s not a failure of process - that’s a failure of courage. Someone’s got to be the one to say ‘stop’ - and if that person is scared of losing their job, you already lost. The real villains aren’t the production managers. They’re the CEOs who think ‘efficiency’ means cutting the people who say no. And the auditors who let it slide because ‘it’s just a small company.’ You don’t get to call yourself safe if your QA team has to ask permission to do their job.
Also - third-party QA? That’s just hiring someone else to take the blame. Same problem, different name.
Brenda King
I’ve trained 30+ QA folks over the years. The ones who last? They’re not the smartest. They’re the ones who learned how to say ‘I need to see the data’ without sounding like a jerk. And they all had one thing: direct access to the CEO. No filters. No ‘let me run it by production first.’ If you’re waiting for approval to say no - you’re already broken. Also - if your QA team doesn’t get invited to the budget meeting? They’re not independent. They’re decoration. ❤️
Keith Helm
Per 21 CFR 211.22, the Quality Assurance Unit must be functionally independent. The term ‘independent’ is defined in 21 CFR 210.3(b)(10) as ‘not under the direct supervision of the manufacturing unit.’ This is not a suggestion. It is a codified requirement. Non-compliance constitutes a violation of Current Good Manufacturing Practice and subjects the facility to regulatory action. I have reviewed 147 FDA Form 483s in the last year. 89% cited this exact deficiency. The data is unambiguous.
Ryan Riesterer
Interesting how the industry conflates ‘independence’ with ‘organizational separation.’ In nuclear, the IAEA’s approach is more sophisticated: it’s about ‘functional independence’ - the ability to act without fear or favor, regardless of reporting line. The real issue isn’t who signs the org chart - it’s whether the QU has access to unfiltered data, authority to halt operations, and protection from reprisal. Many ‘independent’ QUs are still neutered by budget control, performance metrics tied to production KPIs, or ‘collaborative’ culture mandates that punish dissent. The structure is symbolic. The power is illusory.
AI can flag anomalies. But only a human with institutional armor can say ‘no’ to a $2M batch. That’s the real bottleneck - not headcount.
shivani acharya
Ohhh so now we’re gonna put a whole department between the people who make stuff and the people who sell it? Classic. Meanwhile, the CEO’s cousin is running the line, the QA guy is his college roommate, and the ‘independent’ auditor gets a free vacation every year to ‘review’ the plant. This whole system is rigged. The FDA doesn’t care - they’re too busy taking money from pharma lobbyists. You think they’re gonna shut down a plant that’s giving them campaign donations? Please. This post is just corporate theater. The real problem? Greed. Not structure. You can put QA on the moon and if the boss wants to cut corners, he’ll find a way. This isn’t about rules - it’s about who’s paying the bribes.
Write a comment