When you pick up a prescription, you might see a different pill than the one you’ve been taking for years. It’s the same drug, same active ingredient, same dose-but the color, shape, and even the name on the label are different. That’s a generic drug. And if you’ve ever wondered whether it works just as well, the answer isn’t just "yes"-it’s backed by strict science, decades of data, and a regulatory system designed to make sure absorption rates between generics and brand-name drugs are nearly identical.
What Does "Absorption Rate" Even Mean?
Absorption rate isn’t about how strong the drug is. It’s about how fast and how much of the drug actually gets into your bloodstream. Two drugs can have the same amount of active ingredient, but if one gets absorbed slower or less completely, it might not work the same way. That’s why regulators don’t just check the pill’s weight or chemical purity-they measure two key numbers: AUC and Cmax.
AUC (Area Under the Curve) tells you the total amount of drug your body is exposed to over time. Think of it as the total dose you absorb from start to finish. Cmax (Maximum Concentration) shows the highest level the drug reaches in your blood-this tells you how quickly it gets absorbed. Together, these two numbers give a full picture of whether the generic drug behaves like the brand-name version inside your body.
The 80-125% Rule: Not a Range for Failure, But a Standard for Precision
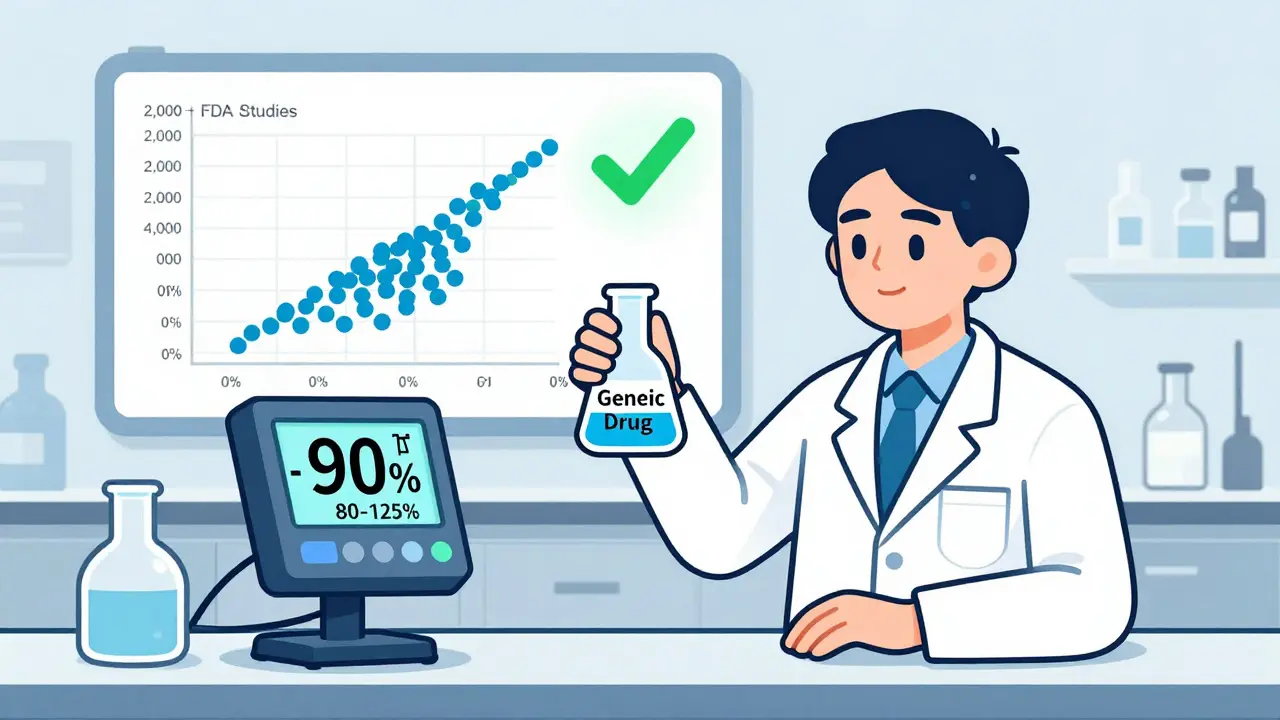
The U.S. Food and Drug Administration (FDA) requires that generic drugs deliver between 80% and 125% of the brand-name drug’s absorption. That sounds like a big gap-45 percentage points!-but it’s not a license for wild variation. This range is based on how much your own body naturally varies from one dose to the next. Even if you take the same brand-name pill twice, your absorption might jump by 10-15% due to digestion, food, or even stress.
The real rule? The 90% confidence interval for the average difference between generic and brand must fall entirely within 80-125%. That’s not a guess. It’s a statistical guarantee. And here’s what the data shows: out of more than 2,000 bioequivalence studies submitted to the FDA, the average difference in absorption was just 3.5% for AUC and 4.35% for Cmax. In nearly 98% of cases, the generic differed by less than 10%. That’s not just close-it’s nearly identical.
Why Do Some People Say Generics Don’t Work the Same?
Every so often, someone posts online: "I switched to the generic and I didn’t feel the same." These stories get shared, amplified, and turned into fear. But they rarely reflect reality.
For example, Drugs.com analyzed over 1,200 patient reviews and found that 12% reported differences. But the biggest complaints? Generic levothyroxine (for thyroid) and generic bupropion (for depression). These are drugs with very narrow therapeutic windows-small changes in blood levels can matter. That’s why the FDA requires tighter standards for them: 90-111% for AUC, not 80-125%.
Even then, the evidence doesn’t support widespread problems. A 2023 meta-analysis of 47 studies with nearly 10,000 patients found no meaningful difference in outcomes between generic and brand-name cardiovascular drugs. The FDA has documented only 12 cases of potential therapeutic failure out of more than 14,000 approved generics between 2008 and 2023. That’s a 0.08% failure rate.
So why do people think they’re different? Sometimes it’s the pill’s appearance. A blue capsule feels different from a white tablet. Sometimes it’s the placebo effect-expecting a cheaper drug to be weaker. Sometimes it’s switching between different generic manufacturers. A 2014 study found that 54% of generic products showed different dissolution rates in the lab. That means the pill breaks down slower or faster in your stomach. But here’s the catch: even if dissolution differs, the body still absorbs the same amount of drug. As long as AUC and Cmax are within range, it works.
Dissolution vs. Absorption: A Common Misunderstanding
Some generic pills dissolve differently in a test tube than the brand. A generic nifedipine might take longer to break apart. A generic amoxicillin might dissolve faster. But dissolution is just a lab test. It doesn’t predict what happens in your body.
The body is messy. Stomach acid, food, gut motility, even the time of day-all these things affect how a drug is absorbed. A pill that dissolves slowly in a lab might still release its full dose in your intestines. And if the drug ends up in your bloodstream at the same level and timing as the brand, it doesn’t matter how fast it broke apart in a beaker.
That’s why the FDA doesn’t rely on dissolution alone. It requires real human studies. Healthy volunteers take both the brand and the generic, blood samples are drawn over hours, and the data is analyzed with strict statistical rules. If the numbers match, the drug gets approved.
What About Narrow Therapeutic Index Drugs?
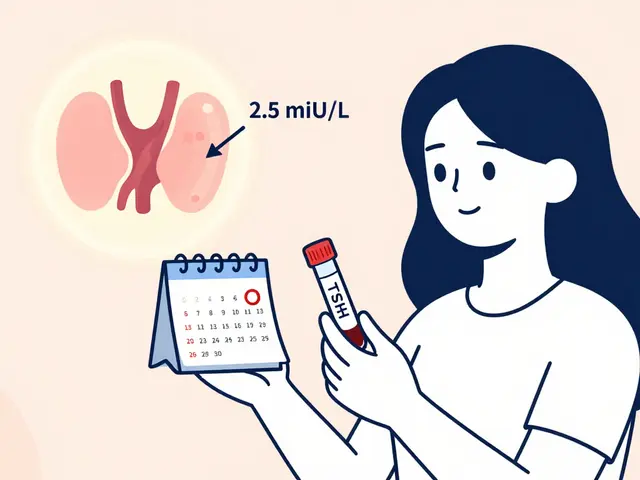
Some drugs are like tightrope walkers. Too little and they don’t work. Too much and they’re toxic. These include warfarin (blood thinner), digoxin (heart drug), phenytoin (seizure drug), and levothyroxine (thyroid hormone). For these, the FDA requires tighter bioequivalence limits: 90-111% for AUC. That’s because even a 10% difference can matter.
That’s why doctors often prefer you stay on the same generic brand once you’re stabilized. If you’ve been on one manufacturer’s levothyroxine for months and your TSH levels are perfect, switching to another generic-even one that meets FDA standards-might cause small fluctuations. It’s not because the new one is bad. It’s because your body got used to a specific formulation. For these drugs, consistency matters more than cost.
How Are Generics Approved? The Real Process
Generic manufacturers don’t just copy the brand. They have to prove they’re the same. Here’s how:
- They identify the brand-name drug as the "reference listed drug."
- They conduct a crossover study with 24-36 healthy volunteers. Each person takes both the generic and the brand, in random order, with a washout period in between.
- Blood samples are taken over 24-72 hours to measure AUC and Cmax.
- Statistical analysis confirms the 90% confidence interval for the ratio of geometric means falls within 80-125% (or 90-111% for narrow drugs).
- The FDA reviews the data. If it passes, the generic is approved.
And once approved? The FDA keeps watching. Through its Adverse Event Reporting System, they track side effects and unexpected outcomes. If problems pile up, they can require new studies or pull the drug.
Why Do Generics Look Different?
U.S. trademark law says a generic can’t look exactly like the brand. That’s why your generic pill might be orange instead of blue, oval instead of round, or have a different imprint. These changes are purely cosmetic. They don’t affect absorption, potency, or safety. The active ingredient is the same. The excipients (fillers, binders) might differ slightly, but they’re chosen to ensure the drug dissolves properly and doesn’t interact with the active ingredient.
And yes-different generic manufacturers can make pills that look and feel different. But if both meet FDA bioequivalence standards, they’re equally safe and effective.
What Does This Mean for You?
If you’re taking a regular medication-antibiotics, blood pressure pills, cholesterol drugs, antidepressants-you can trust the generic. The science is solid. The data is overwhelming. The cost savings? Huge. Generics make up 90% of prescriptions in the U.S. but only 23% of total drug spending. That’s billions saved every year.
For narrow therapeutic index drugs, talk to your doctor. If you’re stable on a brand, staying on it might be best. But if you’re switching for cost, don’t panic. The FDA’s stricter limits for these drugs mean the risk is minimal.
And if you notice a change after switching? Don’t assume it’s the drug. Talk to your pharmacist. Check if you switched manufacturers. Consider whether you’ve changed your diet, sleep, or stress levels. The pill itself is likely not the problem.
The Global Picture
The FDA’s 80-125% standard isn’t just American. The European Medicines Agency (EMA) uses the same rule. Japan’s PMDA is stricter-85-115% for some drugs-but the global consensus is clear: bioequivalence works. The system isn’t perfect, but it’s one of the most rigorously tested in medicine.
And the future? The FDA is moving toward using computer modeling and simulation to predict absorption without always needing human trials. For complex drugs like inhalers or topical creams, new methods are being developed. But the core principle remains: if the drug gets into your bloodstream the same way, it works the same way.
Are generic drugs really as effective as brand-name drugs?
Yes, when approved by the FDA, generic drugs must deliver the same amount of active ingredient into the bloodstream at the same rate as the brand-name version. Bioequivalence studies show the average difference in absorption is typically less than 5%, making them clinically equivalent for most patients.
Why do some people say generics don’t work as well?
Perceived differences often come from changes in pill appearance, switching between generic manufacturers, or psychological factors like expecting a cheaper drug to be less effective. For drugs with narrow therapeutic windows (like levothyroxine), even small variations can matter-but these cases are rare and tightly regulated. In most cases, the drug itself isn’t the issue.
Can different generic brands of the same drug be different?
Yes, different manufacturers may use different fillers or manufacturing processes, which can affect how fast the pill dissolves in the lab. But as long as the AUC and Cmax values fall within the FDA’s 80-125% range, the drug will be absorbed the same way in your body. The FDA requires this for every approved generic.
What are narrow therapeutic index drugs, and why do they need special attention?
These are drugs where small changes in blood levels can cause serious side effects or treatment failure. Examples include warfarin, digoxin, phenytoin, and levothyroxine. For these, the FDA requires a tighter bioequivalence range of 90-111% for AUC. If you’re stable on one version, your doctor may recommend staying on it to avoid small fluctuations.
Is it safe to switch between brand-name and generic drugs?
For most medications, yes. The FDA approves generics only after proving they’re bioequivalent. But for drugs with narrow therapeutic windows, switching manufacturers-even between two approved generics-can cause small changes in blood levels. If you’re doing well on one, it’s often safer to stick with it unless your doctor advises otherwise.
Final Thought: Trust the Data, Not the Myths
Generics aren’t "second-best." They’re the result of a system built on real science, not guesswork. The 80-125% rule isn’t a loophole-it’s a precision tool. And the data? It’s clear. Millions of people take generics every day. They get better. They stay healthy. They save money. And the science says: it works.


Comments
1 Comments
Adam Short
Let me get this straight - we’re told to trust these generics because some statistician in a lab says the numbers are "within range"? Meanwhile, my cousin took a generic version of his blood pressure med and ended up in the ER because his heart went haywire. Yeah, sure, 98% of cases are fine - but what about the 2%? You think they care about your confidence intervals when they’re gasping for air? The FDA doesn’t know my body. The lab doesn’t know my body. Only my body knows what it can handle. And if I want the brand that’s been working for 12 years? I’ll pay for it. No one’s gonna tell me to gamble with my life because "science" says it’s fine.
Write a comment