Antiviral Flu Treatment Selector
Choose your patient profile
This tool helps identify the most appropriate flu antiviral based on patient characteristics and flu season resistance patterns.
Your selection will appear here
Every flu season, doctors and patients face a quick decision: which antiviral will actually help you recover faster and avoid complications? While many hear only about Tamiflu, there are several other medicines on the market that promise similar or even better results. This guide breaks down Tamiflu (Oseltamivir) and its main rivals, looking at how they work, how effective they are, side‑effects, resistance issues, dosing convenience, and price. By the end you’ll know which drug fits your situation best.
What is Tamiflu (Oseltamivir)?
Tamiflu (Oseltamivir) is an oral neuraminidase inhibitor approved by the FDA to treat and prevent influenza A and B infections. First approved in 1999, it is taken as a capsule or liquid within 48 hours of symptom onset. The drug targets the influenza virus’s neuraminidase enzyme, preventing newly formed viral particles from escaping infected cells.
How Tamiflu Works - The Science Behind It
Influenza viruses use the neuraminidase protein to cleave sialic acid on host cells, a step essential for virus release. By blocking this enzyme, Tamiflu stops the spread of the virus within the respiratory tract. The active form, oseltamivir carboxylate, is generated in the liver mainly by the enzyme CYP2C19. Genetic variations in CYP2C19 can slightly affect drug levels, but for most patients the standard 75mg twice‑daily regimen works well.
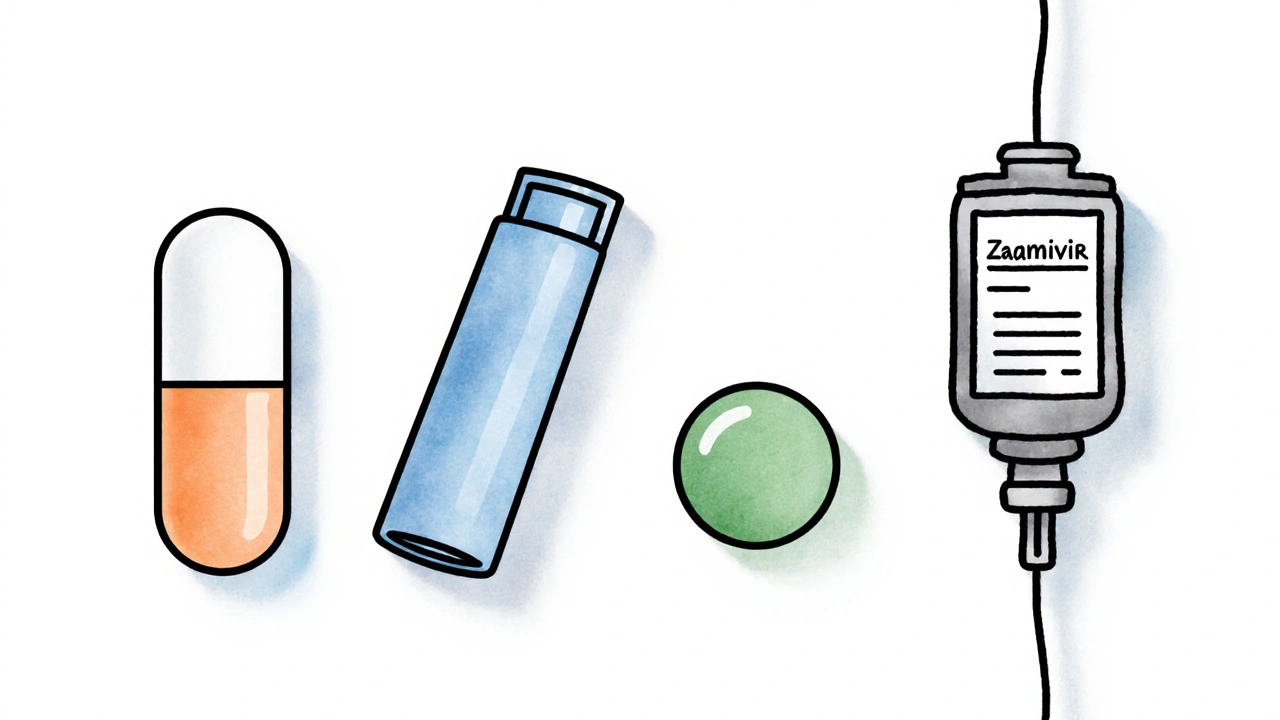
Alternative Flu Antivirals - Who Else Is in the Game?
Zanamivir (Relenza) is an inhaled neuraminidase inhibitor that must be taken twice daily for five days. Unlike Tamiflu, it’s delivered through a small inhaler, making it a good option for patients who can’t swallow pills.
Baloxavir marboxil (Xofluza) is a cap‑dependent endonuclease inhibitor that requires just a single oral dose. It works at an earlier stage of viral replication, which can translate into a faster reduction in viral load.
Peramivir (Rapivab) is an intravenous neuraminidase inhibitor administered as a single infusion in a clinical setting. Its IV route is useful for hospitalized patients who can’t take oral medication.
All these drugs share the goal of limiting influenza replication, but they differ in administration route, dosing schedule, resistance patterns, and cost.
Key Comparison Criteria
To decide which antiviral is right for you, we evaluate five core factors:
- Efficacy: How quickly does the drug reduce fever and viral shedding?
- Resistance: How often do influenza strains develop resistance to the drug?
- Dosing Convenience: Pill, inhaler, single dose, or IV?
- Side‑Effect Profile: Common adverse events and serious risks.
- Cost & Accessibility: Price per treatment course and insurance coverage.
Side‑by‑Side Comparison Table
| Attribute | Tamiflu (Oseltamivir) | Zanamivir (Relenza) | Baloxavir marboxil (Xofluza) | Peramivir (Rapivab) |
|---|---|---|---|---|
| Drug class | Neuraminidase inhibitor | Neuraminidase inhibitor | Endonuclease inhibitor | Neuraminidase inhibitor |
| Administration | Oral capsule or liquid, twice daily for 5 days | Inhaled powder, twice daily for 5 days | Oral single dose | IV infusion, single dose |
| Time to symptom relief | 1-2 days reduction in fever | Similar to Tamiflu, but requires proper inhalation technique | Often 1 day faster than Tamiflu in clinical trials | Rapid reduction in hospitalized patients, but data limited to severe cases |
| Resistance rates (2023‑2024 CDC data) | ~1‑2% for influenza A(H1N1), higher for H3N2 | ~0.5% for A(H1N1), negligible for B | Very low (<0.1%) but emerging mutations reported | ~2% in treated severe cases |
| Common side‑effects | Nausea, vomiting, headache | Cough, bronchospasm (rare), nasal irritation | Diarrhea, mild liver enzyme elevation | Infusion‑related reactions, mild rash |
| Serious adverse events | Rare neuropsychiatric events (mostly in children) | Rare severe bronchospasm | Very rare hepatic injury | Rare anaphylaxis |
| Cost (US, 2025) | $70‑$90 for a 5‑day course | $120‑$150 (inhaler device) | $150‑$180 (single dose) | $300‑$350 (IV administration) |
| Typical patient profile | Outpatient adults, children >1yr, prophylaxis for contacts | Patients comfortable with inhaler, no severe asthma | Patients needing rapid clearance, single‑dose convenience | Hospitalized adults with severe flu |
Choosing the Right Antiviral - Patient Scenarios
CDC recommends
the antiviral that best matches a patient’s age, comorbidities, and ability to tolerate the route of administration. Here are three common scenarios:
- Scenario A - Healthy adult, early symptoms: A 28‑year‑old who spots fever within 24hours can safely use Tamiflu or Baloxavir. If she prefers a single pill and is willing to pay a bit more, Baloxavir cuts the treatment to one dose.
- Scenario B - Asthmatic teen who can’t swallow pills: An 15‑year‑old with mild asthma may struggle with Tamiflu’s nausea. Zanamivir’s inhaled form bypasses the GI tract, but doctors must ensure the teen can use the inhaler correctly to avoid bronchospasm.
- Scenario C - Hospitalized elderly with heart disease: A 72‑year‑old admitted with pneumonia secondary to influenza benefits from Peramivir’s IV delivery, allowing clinicians to ensure adequate drug levels despite potential swallowing difficulties.
In each case, the FDA has approved the indicated uses, but clinicians also weigh resistance trends reported by the CDC each season.
Practical Tips for Patients and Providers
1. Start early. All antivirals are most effective when taken within 48hours of symptom onset.
2. Check for resistance. If you live in an area with known high oseltamivir resistance (e.g., certain H3N2 clades), discuss alternative options.
3. Consider side‑effects. Nausea with Tamiflu can be mitigated by taking it with food; inhaled Zanamivir may aggravate asthma.
4. Review insurance coverage. Some plans classify Baloxavir as a specialty drug, increasing out‑of‑pocket costs.
5. Don’t self‑prescribe. A proper diagnosis by a healthcare professional ensures you get the antiviral best suited to the circulating strain.
Key Takeaways
- Tamiflu remains a solid first‑line oral option for most out‑patients.
- Zanamivir is useful for those who can’t tolerate oral meds but have no severe asthma.
- Baloxavir offers a single‑dose convenience and may work faster, but costs more.
- Peramivir is reserved for hospitalized patients needing IV therapy.
- Local resistance patterns, side‑effect profiles, and dosing convenience drive the final choice.
Frequently Asked Questions
Can I take Tamiflu if I’m pregnant?
The CDC classifies Tamiflu as a Category C drug for pregnancy. This means risk cannot be ruled out, but the benefits may outweigh potential harms if a pregnant woman contracts flu. Always discuss with your obstetrician before starting.
Is a single dose of Baloxavir enough for severe flu?
Baloxavir is approved for uncomplicated influenza. For severe cases or hospitalized patients, clinicians usually add IV antivirals like Peramivir or supportive care.
Why do some people get nausea with Tamiflu?
Oseltamivir is absorbed in the gastrointestinal tract, and its active metabolite can irritate the stomach lining. Taking the capsule with food or using the liquid formulation mixed with juice often reduces nausea.
How do I know if my flu strain is resistant to Tamiflu?
Resistance testing isn’t routine for everyday patients. Public health labs, however, publish seasonal resistance reports. If your doctor knows that a high‑resistance strain is circulating, they may prescribe an alternative upfront.
Can I use over‑the‑counter meds together with Tamiflu?
Yes, acetaminophen or ibuprofen for fever and aches are safe alongside Tamiflu. Just avoid other neuraminidase inhibitors, as combining them offers no extra benefit and could raise side‑effect risk.


Comments
11 Comments
Malia Rivera
When we talk about beating the flu, we must first consider the resilience of our own healthcare infrastructure. A drug like Tamiflu, produced domestically, embodies the very spirit of self‑reliance that keeps America strong. While newer antivirals glitter with novelty, they often depend on foreign patents and supply chains that can be disrupted. In the end, the tried‑and‑true oral capsule remains the pragmatic choice for the everyday citizen.
Cindy Thomas
Actually, the hype around Baloxavir is overblown - a single dose sounds great until you realize resistance can spike in a month. 😒 The old Oseltamivir still outperforms it in real‑world settings.
James Falcone
Give me Tamiflu any day, it’s cheap and reliable.
Frank Diaz
One must question the very premise that newer equals better; history teaches us that convenience rarely outweighs efficacy. Oseltamivir’s metabolic pathway has been studied for decades, offering a predictable safety profile that experimental agents lack. Moreover, the societal cost of an unfamiliar drug failing mid‑season is a risk we cannot ignore. Let us not be seduced by novelty at the expense of proven outcomes.
lisa howard
The pandemic fatigue that has settled over the nation makes the choice of flu antivirals feel like a theatrical showdown between the old guard and the avant‑garde.
On the one hand we have Tamifuz, the stalwart whose capsule has been popping in medicine cabinets since the cusp of the millennium, promising a reliable, twice‑daily regimen that most patients can manage without a second thought.
On the other hand sit the sleek newcomers-Zanamivir with its whiff of powdered inhalation, Baloxavir flaunting a single, heroic dose, and Peramivir delivering an IV drip that feels more at home in an intensive care unit than a suburban pantry.
Each of these agents carries its own mythos, yet the underlying science remains rooted in the same viral life cycle, merely tugging at different molecular ropes.
The neuraminidase inhibitors, Tamiflu, Zanamivir and Peramivir, all aim to cripple the virus’s escape hatch, while Baloxavir takes a bold detour, snipping the viral RNA’s transcription machinery.
This distinction is not academic fluff; it translates into how quickly fevers break, how soon patients can return to work, and whether families avoid the dreaded “flu‑week” that wipes out weekend plans.
Consider also the practicalities-an inhaler demands proper technique, a single dose may be pricey, and an IV infusion demands a hospital bed that could be occupied by a COVID patient.
The side‑effect spectrum, too, is an intricate tapestry where nausea, bronchospasm, and rare hepatic irritations flutter like warning flags on a battlefield.
Resistance patterns, ever‑shifting like political alliances, have shown Tamiflu’s H3N2 strains occasionally mutating, while Zanamivir’s resistance remains a whisper in the wind.
Cost, that ever‑present villain, sees Baloxavir perched at a premium, whereas generic Oseltamivir can be sourced for a fraction of the price, a fact that resonates deeply with cash‑strapped households.
From a public‑health perspective, the ease of prescribing a pill that patients can take at home cannot be overstated, especially when clinics are overwhelmed.
Nonetheless, the single‑dose elegance of Baloxavir offers a seductive promise of compliance, a factor that could tilt the scales in favor of those who notoriously forget to finish a five‑day course.
In the end, the decision matrix is a mosaic of efficacy, convenience, side‑effects, resistance, and wallet‑impact, each piece demanding careful scrutiny.
As clinicians weigh these variables, they must also listen to the patient’s personal circumstances-whether they can manage an inhaler, afford a premium drug, or need rapid viral clearance due to a demanding job.
Only then can the chosen antiviral truly become a tailored shield against the relentless march of influenza.
Valerie Vanderghote
While you paint a vivid tableau of the antiviral arena, it’s worth highlighting that patient adherence often crumbles under the weight of side‑effects we casually mention. Nausea from Tamiflu can force a dose reduction, and the inhaler’s powder can trigger bronchospasm in otherwise stable asthmatics. Moreover, insurance formularies sometimes block the “single‑dose hero” of Baloxavir, leaving patients stranded with generic options that feel like a compromise. The real‑world data from community clinics show a surprising drop‑off after the second day of a five‑day Tamiflu course, which undermines the theoretical efficacy you outlined. In addition, the IV logistics of Peramivir translate into higher systemic costs that many outpatient settings cannot bear. Cost‑effectiveness analyses reveal that, when you factor in the hidden expenses of monitoring and administration, the cheap pill still often wins the economic battle. Ultimately, the decision matrix you propose must also integrate these pragmatic hurdles, otherwise it remains a beautiful but impractical portrait. So, clinicians, arm yourselves with not just the data, but also the realities of the patients’ daily lives.
Kate Marr
America’s own production lines keep Tamiflu readily available 🇺🇸, making it a reliable go‑to for most families. Baloxavir may be flashy, but the cost and limited access can be a deal‑breaker 💸.
Mary Davies
But what about those who can’t afford the premium price tag? Is the single‑dose magic worth the financial strain on a middle‑class household?
Michael Dalrymple
When faced with influenza, the priority should be timely initiation of therapy, as early treatment consistently shortens the disease course. Evaluating each antiviral’s pharmacokinetics, safety profile, and patient suitability will guide optimal selection. Encourage patients to discuss any concerns about side‑effects or administration routes with their healthcare provider. By aligning clinical evidence with individual circumstances, we can maximize therapeutic benefit while minimizing complications.
Emily (Emma) Majerus
Thx for the tipz, I'll hop on the phone 2 my doc now.
Virginia Dominguez Gonzales
Indeed, the art of medicine lies in marrying science with compassion; let us champion both the data‑driven choices and the human stories behind each prescription. 🌟
Write a comment