Every year, millions of people in the U.S. face a simple but powerful choice at the pharmacy counter: take the brand-name drug they recognize, or switch to a cheaper generic version. For many, the decision isn’t just about price-it’s about trust, fear, and confusion. You’ve been prescribed a medication. The doctor says it’s fine. The pharmacist hands you a pill that looks completely different. Is it the same? Will it work? Should you pay more for the name you know?
What Exactly Is a Generic Drug?
A generic drug is not a copy. It’s not a knockoff. It’s the exact same medicine, chemically speaking. The active ingredient-the part that actually treats your condition-is identical to the brand-name version. If your doctor prescribed atorvastatin, the generic version of Lipitor, you’re getting the same molecule that lowers cholesterol. The FDA requires this. No exceptions.
The difference lies in everything else: the color, the shape, the name on the pill, the filler ingredients. Generics don’t need to repeat the expensive clinical trials that brand-name companies ran to get approval. Instead, they prove they deliver the same amount of medicine into your bloodstream at the same rate. This is called bioequivalence. The FDA demands that the 90% confidence interval for absorption falls between 80% and 125% of the brand. In practice, most generics are within 3-4%-almost identical.
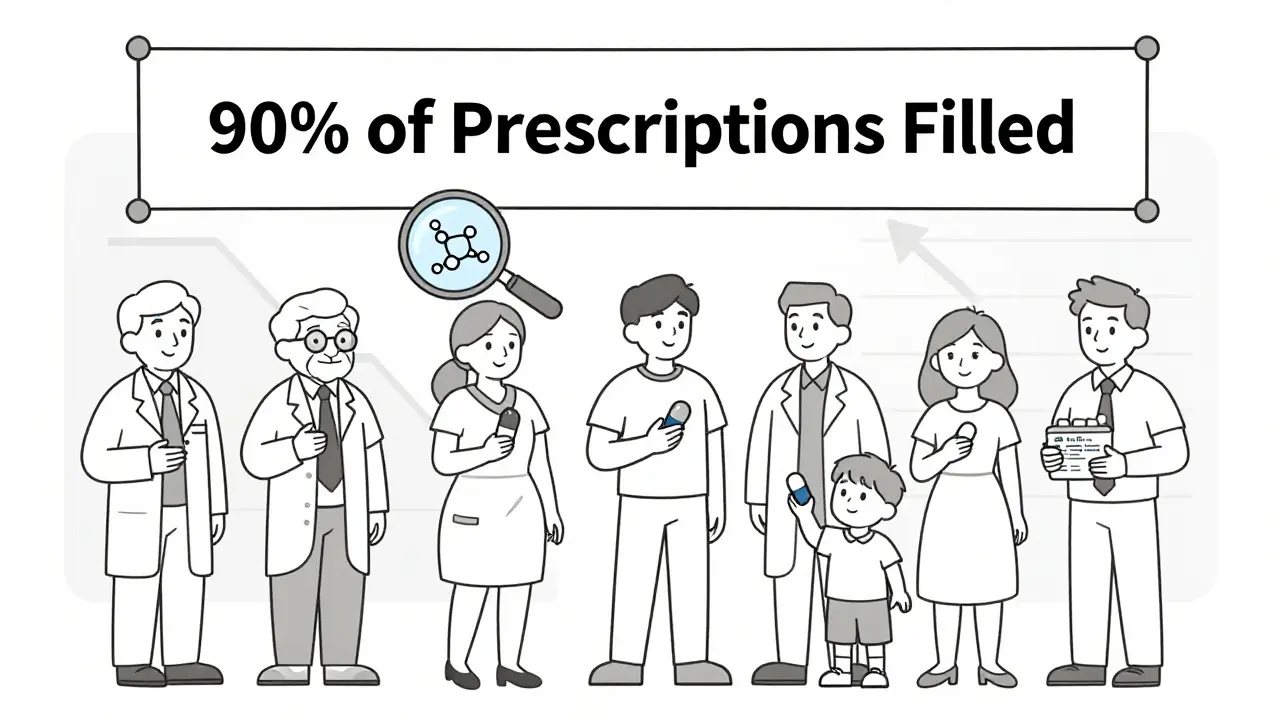
Since 1984, the Hatch-Waxman Act created this system. It allowed generics to enter the market after patents expired, without repeating every test. The result? Today, 90% of all prescriptions filled in the U.S. are generics. But they make up only 23% of total drug spending. That’s because they cost far less.
How Much Money Do You Actually Save?
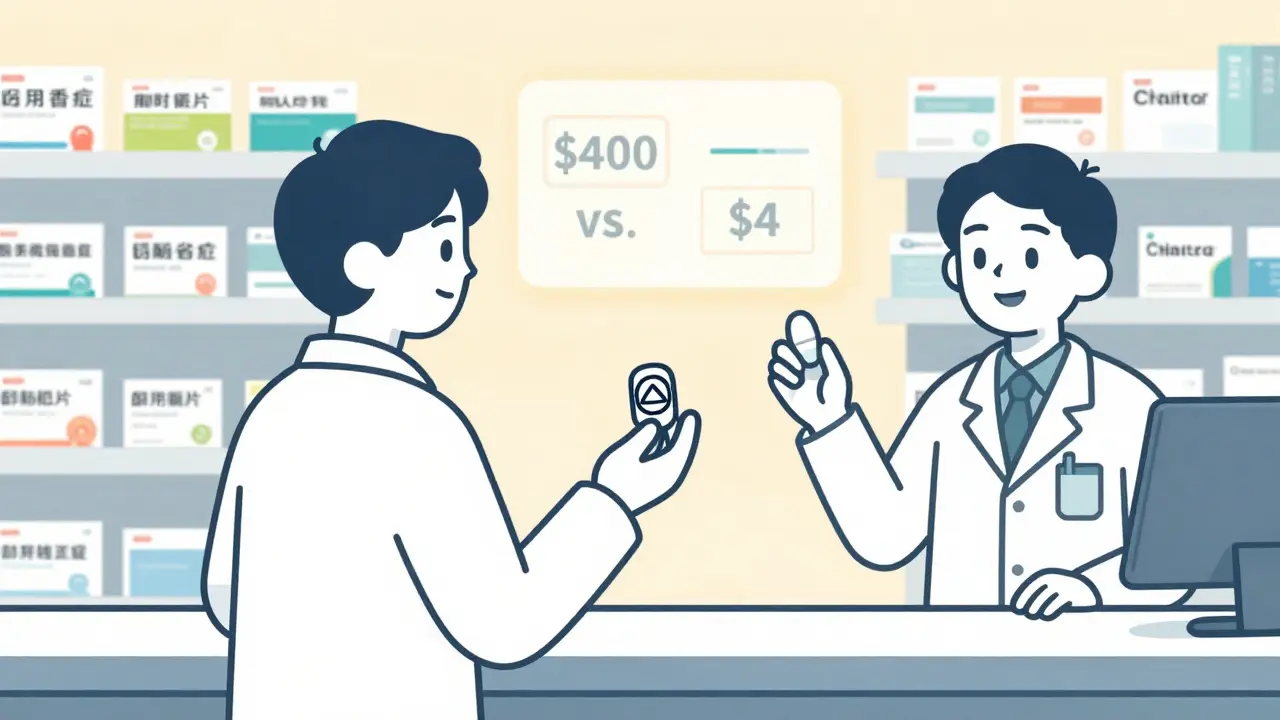
The numbers speak for themselves. Sertraline, the generic version of Zoloft, costs about $4 for a 30-day supply. The brand? Around $400. Atorvastatin (Lipitor generic) runs $0.10 per tablet. The brand? $4.50. That’s a 98% drop in price.
Over the last decade, generic drugs saved the U.S. healthcare system $1.67 trillion. That’s $265 saved per person every year. For someone on a fixed income, that’s not just convenience-it’s survival. A 2021 Kaiser Family Foundation study found that patients who couldn’t afford their brand-name meds were 29% more likely to skip doses. When switched to generics, that number dropped to 14%.
Some brands have started offering their own generics-called “authorized generics.” Eli Lilly’s Humalog insulin, for example, has a generic version sold under the same name but at 20-30% less than the original brand. It’s the same formula, same packaging, same manufacturer. Just cheaper.
When Should You Stick With the Brand?
Most of the time, generics are perfectly safe and effective. But there are exceptions. The FDA and medical experts agree: for drugs with a narrow therapeutic index, even tiny differences in how the body absorbs the medicine can matter.
Examples include:
- Levothyroxine (for hypothyroidism): Small changes in thyroid hormone levels can cause fatigue, weight gain, or heart problems. In 28 states, pharmacists must notify the doctor before switching brands.
- Warfarin (a blood thinner): Even a 5% variation in absorption could increase clotting or bleeding risk. Studies of over 100,000 patients found no major difference with generics, but doctors often prefer consistency.
- Carbamazepine (for epilepsy): A 2017 study in Neurology found a small but real number of patients had breakthrough seizures after switching to a different generic version.
- Extended-release formulations: Drugs like Wellbutrin XL or Adderall XR rely on complex coatings to release medicine slowly. Some generics have had issues with inconsistent release rates. The FDA issued a warning in 2012 about certain generic bupropion products.
- Inhalers and patches: A 2016 study in Chest found that 12% of patients switching from Advair Diskus to a generic version struggled with the inhaler’s mechanism-even though the active ingredients were identical.
If you’re on one of these medications, talk to your doctor before switching. Don’t assume it’s safe just because it’s cheaper.
Why Do Generics Look Different?
It’s not a trick. It’s the law. Brand-name companies trademark the look of their pills-color, shape, logo. Generics can’t copy that. So they change it. One month you get a blue oval. The next, a white capsule. That’s normal.
But this causes confusion. A 2023 study in Patient Education and Counseling found that 27% of patients on Reddit and Drugs.com reported anxiety or mistakes because their pill changed appearance. One woman thought her new generic thyroid pill was a different drug and stopped taking it. Her TSH levels spiked.
Simple fix: Keep a list of your meds with their appearance. Use the FDA’s Drugs@FDA database. Or ask your pharmacist for a photo of the pill before you leave the pharmacy. Many now have digital pill identifiers on their screens.
What Do Patients Really Think?
On Drugs.com, 82% of users rated generics as effective. That’s only 3% lower than brand-name drugs. But the complaints tell a different story. People don’t complain about effectiveness-they complain about:
- “I switched to generic and felt weird for a week.” (Often placebo effect or unrelated change)
- “The new pills are smaller and harder to swallow.” (Inactive ingredients changed)
- “I don’t trust it because it’s cheaper.” (Fear, not evidence)
But when patients are educated-told how the FDA tests generics-89% continue using them. And adherence improves by 22% because they can actually afford the medicine.
On Reddit’s r/Pharmacy, users share stories of switching from $650/month Lyrica to $15/month pregabalin and seeing zero difference in pain control. On r/PersonalFinance, 93% of positive comments mention cost as the reason for choosing generics.
What Should You Do?
Here’s a practical guide:
- Ask your doctor: “Is this medication one where switching to generic could be risky?” If it’s for high blood pressure, depression, or diabetes, the answer is almost always no.
- Check the FDA’s Orange Book: Look up your drug. If it’s rated “AB,” it’s considered interchangeable.
- Use GoodRx: Compare prices. Sometimes the brand is cheaper than a generic due to coupons.
- Stick with one pharmacy: They track which manufacturer you get. If your generic switches from one company to another, they can alert you.
- Don’t panic over appearance changes: Call your pharmacist. Ask what changed. Most times, it’s nothing.
- Monitor yourself: If you’re on a narrow therapeutic index drug, track symptoms. If you feel off, call your doctor-not because the drug failed, but because your body might need an adjustment.
The Bigger Picture
Generics aren’t just a cost-cutting tool. They’re a public health win. Without them, millions would skip doses, end up in the ER, or go bankrupt. The U.S. spends more on healthcare than any other country-and generics are one of the few things keeping it from collapsing under its own weight.
Pharmaceutical companies spend billions marketing brand names. But the science doesn’t lie: 98.5% of the time, generics work just as well. The 1.5% where they don’t? That’s not because generics are inferior. It’s because some drugs are inherently tricky to replicate perfectly.
The real question isn’t “Which is better?” It’s “Which is right for me?” For most people, the answer is generic. For a small number, the answer is brand. But you won’t know unless you ask, learn, and stay informed.
Medicine isn’t about brands. It’s about results. And for the vast majority of patients, generics deliver the same results-without the same price tag.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for safety, strength, purity, and quality as brand-name drugs. They must prove bioequivalence-meaning they deliver the same amount of active ingredient into your bloodstream at the same rate. The FDA inspects manufacturing facilities for both types of drugs, and compliance rates are nearly identical: 98.7% for generics versus 99.1% for brands.
Why do generic pills look different from brand-name pills?
By law, generic manufacturers cannot copy the exact appearance (color, shape, logo) of brand-name pills because those are trademarked. So generics look different to avoid legal issues. But the active ingredient is the same. If your pill changes shape or color, it’s usually just a switch between different generic manufacturers-not a change in effectiveness.
Can switching to a generic drug cause side effects?
Side effects from switching are rare and usually not caused by the active ingredient. They may happen if you’re sensitive to a different inactive ingredient (like dye or filler) in the generic version. Some people report feeling “different” after switching-this is often psychological or due to unrelated changes. For drugs with narrow therapeutic indices (like thyroid meds or blood thinners), even small absorption differences can matter. Always monitor how you feel and report changes to your doctor.
Is it true that some generics don’t work as well?
In over 98% of cases, generics work just as well. But there are exceptions with complex drug delivery systems-like extended-release tablets, inhalers, or patches. For example, some generic versions of Advair Diskus or EpiPen had device differences that affected how patients used them, not the medicine itself. The FDA has issued warnings for specific generic products in the past, but these are rare and publicly listed. Always check the FDA’s Orange Book for therapeutic equivalence ratings.
Should I always choose the cheapest generic?
Usually yes-but not always. For most medications, any FDA-approved generic is fine. But for drugs like levothyroxine or warfarin, consistency matters. If you’ve been stable on one generic brand, stick with it. Switching between different generic manufacturers can cause small fluctuations. Talk to your pharmacist about keeping the same manufacturer if possible. Also, sometimes the brand-name drug is cheaper than a generic due to coupons or insurance deals-always compare prices with GoodRx.
Comments
13 Comments
Jennifer Littler
Let’s be real-the FDA’s bioequivalence standards are ridiculously tight. 80-125%? That’s not ‘close enough,’ that’s ‘you’re literally the same molecule.’ I’ve reviewed bioequivalence data for a dozen generics in my pharmacoeconomics work. The variance is often less than 2%. People panic over pill color like it’s a witch’s brew.
Alfred Schmidt
Oh great, another corporate shill article. So what? You’re telling me I should trust some Chinese factory’s white pill over the $500 brand I’ve been on for 10 years? My body knows the difference. I don’t care what your ‘science’ says-I feel it. And I’m not some lab rat for Big Pharma’s cost-cutting schemes.
Priscilla Kraft
My grandma switched from brand-name Synthroid to generic and cried because the pill was smaller 😭 She thought she was getting ‘fake medicine.’ We sat down, showed her the FDA page, and she now calls generics ‘the quiet heroes.’ 💙
Sam Davies
Oh, so now we’re pretending generics are some kind of egalitarian miracle? How quaint. The FDA’s 80-125% window is laughably broad-it’s not ‘identical,’ it’s ‘close enough for regulatory convenience.’ And don’t get me started on the fact that 80% of generics are manufactured in India and China, where inspection standards are… flexible. I’ll take my $400 Zoloft, thank you very much. At least I know who made it-and that it wasn’t churned out in a facility that once had a rat infestation.
Vincent Clarizio
Let me tell you something about the soul of medicine. It’s not in the molecule-it’s in the ritual. The weight of the bottle in your hand. The logo you recognize. The ritual of swallowing that pill like a sacrament. Generics? They’re pharmaceutical austerity. They strip away dignity. You’re not just buying a drug-you’re buying peace of mind. And peace of mind? That’s not priced in dollars. It’s priced in trust. And trust? You can’t manufacture that in a bulk warehouse in Hyderabad. When you switch to a generic, you’re not just changing the pill-you’re changing your relationship with your own healing. And that’s not just pharmacology. That’s existential.
Christian Basel
Generic bioequivalence? Please. I’ve seen studies where the Cmax varied by 22% between two generic manufacturers of the same drug. That’s not ‘within range,’ that’s a wild swing. And don’t get me started on the fillers-some generics use lactose, others use corn starch. If you’re allergic? Congrats, you just got a mystery anaphylaxis. The FDA doesn’t test for that. They test for ‘active ingredient.’ That’s not medicine. That’s compliance theater.
Alex Smith
People think generics are ‘cheap’ because they’re ‘bad.’ But here’s the real irony: the brand-name companies are the ones who *make* the generics. Eli Lilly makes the generic of Humalog. Pfizer makes the generic of Lipitor. So you’re not avoiding Big Pharma-you’re just avoiding their marketing department. The pill is the same. The factory is the same. The only thing different? The label. And your bank account.
Michael Patterson
generic dont work like brand name i tried it for my anxiety and i felt like i was floating in a vat of lukewarm soup for two weeks. my doctor said its placebo but i know better. the body remembers the brand. the body has memory. and the body hates being tricked.
Matthew Miller
Wow. Another feel-good article that ignores the fact that 40% of generic manufacturers have FDA warning letters. You think the FDA is a guardian? It’s a rubber stamp. And the 90% stat? That’s prescriptions filled, not prescriptions *taken correctly*. People switch generics every month and get confused. They stop taking meds. They die. This isn’t a cost-saving win-it’s a public health landmine.
Madhav Malhotra
In India, generics are the only option for most people. My uncle took generic warfarin for 8 years. No clots. No bleeding. No drama. The real problem isn’t the pill-it’s the fear. We need education, not elitism. Also, the color of the pill? In Mumbai, we don’t care. We care if it works. And it does.
Priya Patel
My mom switched to generic levothyroxine and thought she was dying because the pill was round instead of oval 😅 She called me screaming. We looked up the FDA database together-same active ingredient, same dosage. She now calls it ‘the quiet little pill that saved us $300/month.’ 🙌
Jason Shriner
generics are just brand names with a bad haircut and a discount sticker. i dont trust something that looks like it was made in a basement with a 3d printer. also, why do they always make the pills so small? i can't even swallow them. i feel like i'm swallowing a grain of sand. also, my dog licked one once. he lived. but still.
Sean Feng
Generic works. I’ve been on it for 5 years. Saved $4k a year. No issues. Stop overthinking it.
Write a comment